TEST #2: NAC and Hydrogel
I'm looking for any changes on Live Blood Tests, now using NAC. Specifically, I'm interested in any changes that may occur in the hydrogel and network as a result of using these substances.
Hydrogel. I will not attempt to include various references regarding hydrogels within this article. The field is vast, and as a layperson, I do not definitively know the type of hydrogel present in my blood. Therefore, I will simply mix different supplements into fresh drops of my blood and observe any potential changes.
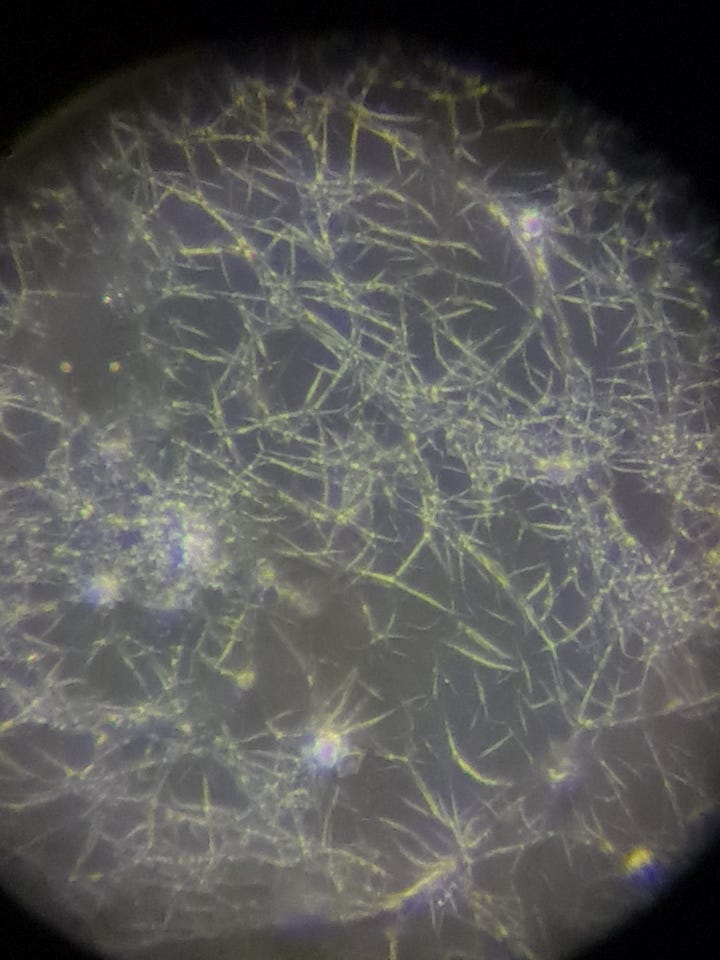
Two phenomena will be monitored: how these supplements affect any hydrogel in my blood, and what occurs with the net-like structure appearing at the microscope slide's edges.
In the previous article, I showed the behavior of the net-like structure with the addition of NAC; the hydrogel was not posted. In this article, I will present the interactions of NAC on the hydrogel, and later demonstrate the effects of Nattokinase, Serrapeptase, and Turpentine on both the hydrogel and the net.
Self-Assembly Hydrogels: Observations on Light and pH Effects
Two main approaches to self-assembly hydrogels have been discussed in various articles: light exposure and/or changes in environmental pH. It is likely that additional factors also influence their behavior.
The response to light can be observed when the microscope light is directed onto the hydrogel. Changes in pH, however, have been observed to affect the structure of the hydrogel, but not its spreading behavior (at least with the supplements studied thus far).
A few potentially interesting references are linked at the bottom of this article. I would like to highlight just one or two sentences here:
Based on the different gelation mechanisms, hydrogels can be classified into chemical hydrogels and physical hydrogels (Mahinroosta et al., 2018).
The dynamic reversible nature of these interactions endows them with self-healing and shear-thinning properties;
It is worth mentioning that not all the Fmoc-modified dipeptides form hydrogels in specific conditions, and most of those dipeptides generally form hydrogels at low pH, …
Although some successful dipeptide hydrogels have been reported, it is still difficult to predict and control the results of molecular assembly, which are derived by various factors that are external (solvent, temperature, light, etc.) and internal (molecular geometry, hydrophobicity, electronics, etc.).
My Experiments
The Base Hydrogel
This is how the base hydrogel appears on the slide without any added supplements.
It can be seen as a clear, gel-like material that begins to spread after being placed on the slide. This spreading continues for about 10 minutes at the speed visible in the video. After that, it spreads at a rate that is no longer visible to the naked eye. It reaches its final size the next day through this slow-spreading process.
Middle of the slide:
At the edge of the slide:
NAC Effects on the Hydrogel
As seen in the previous NAC article, the net-like structure was erased by NAC and did not reappear after 24 hours. The following images show what it did to the hydrogel.
Although NAC erased the net-like structure, after a few minutes the hydrogel started to spread from a specific point on the edge of the slide, seemingly out of nowhere. This spreading can be seen in the second video above.
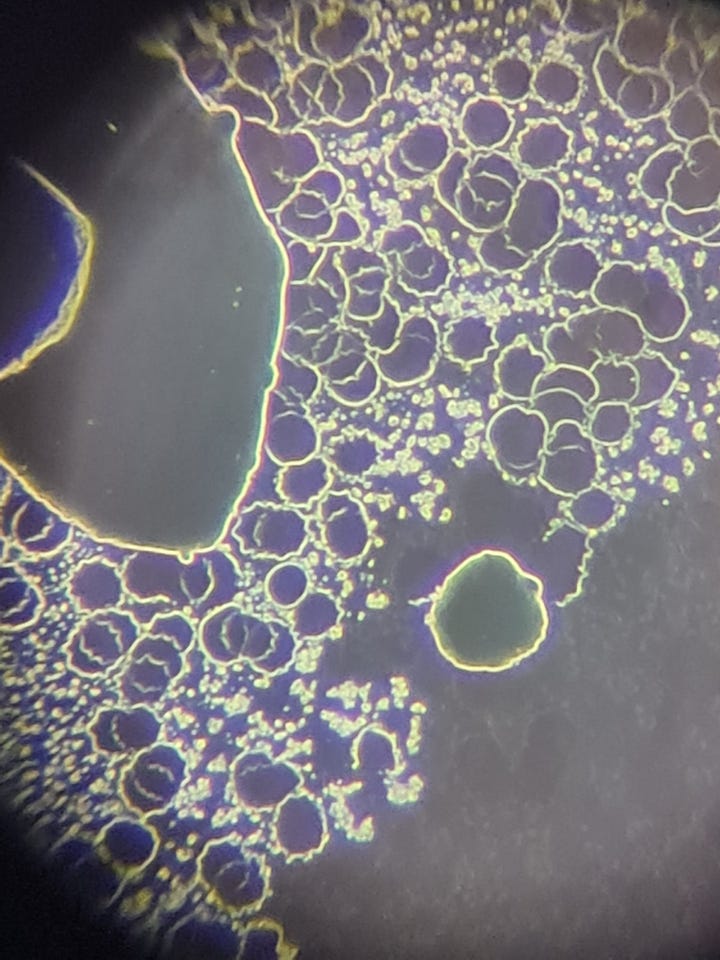
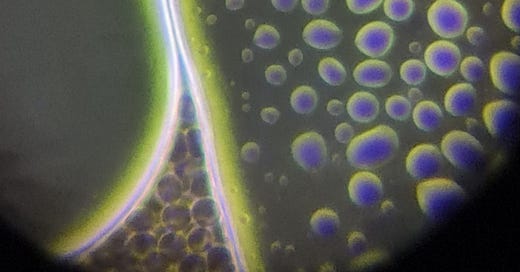
The hydrogels inside the slide looked like this: NAC pH: 3

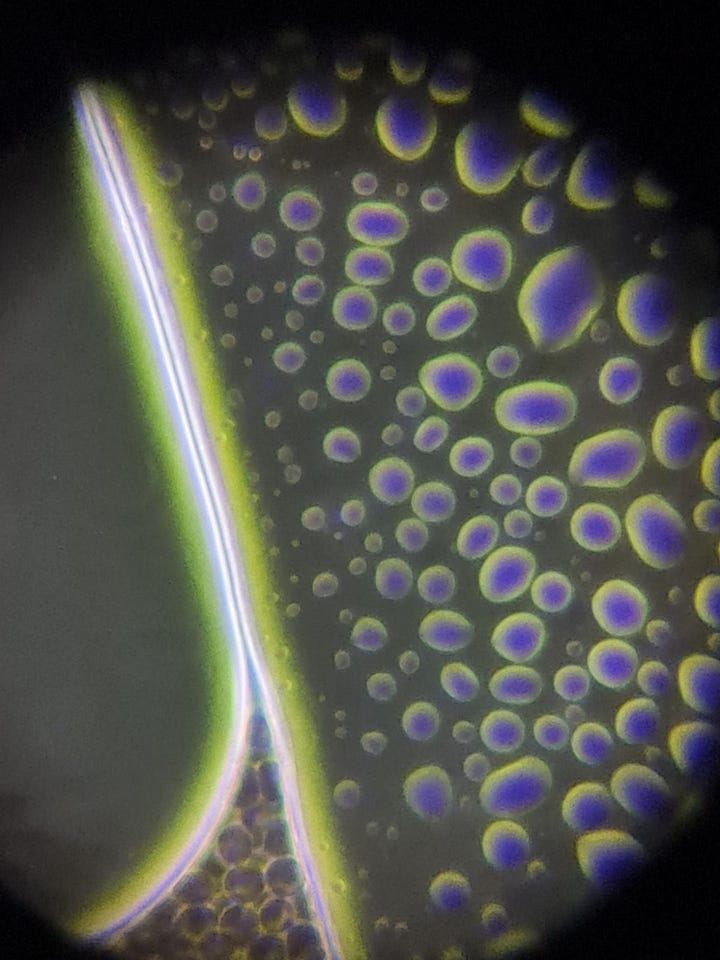
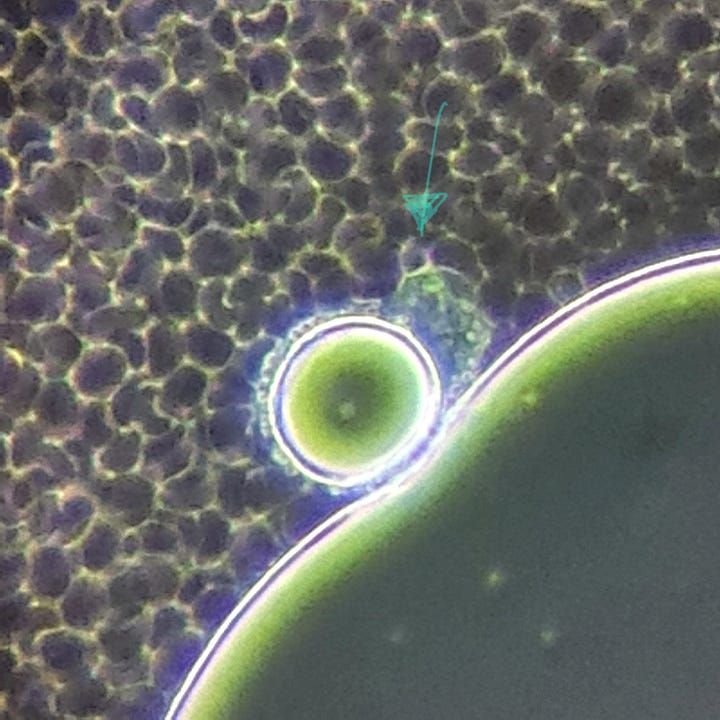
This is a large hydrogel area among RBCs. There are many smaller and larger bubble-like structures on the surface of the hydrogel:
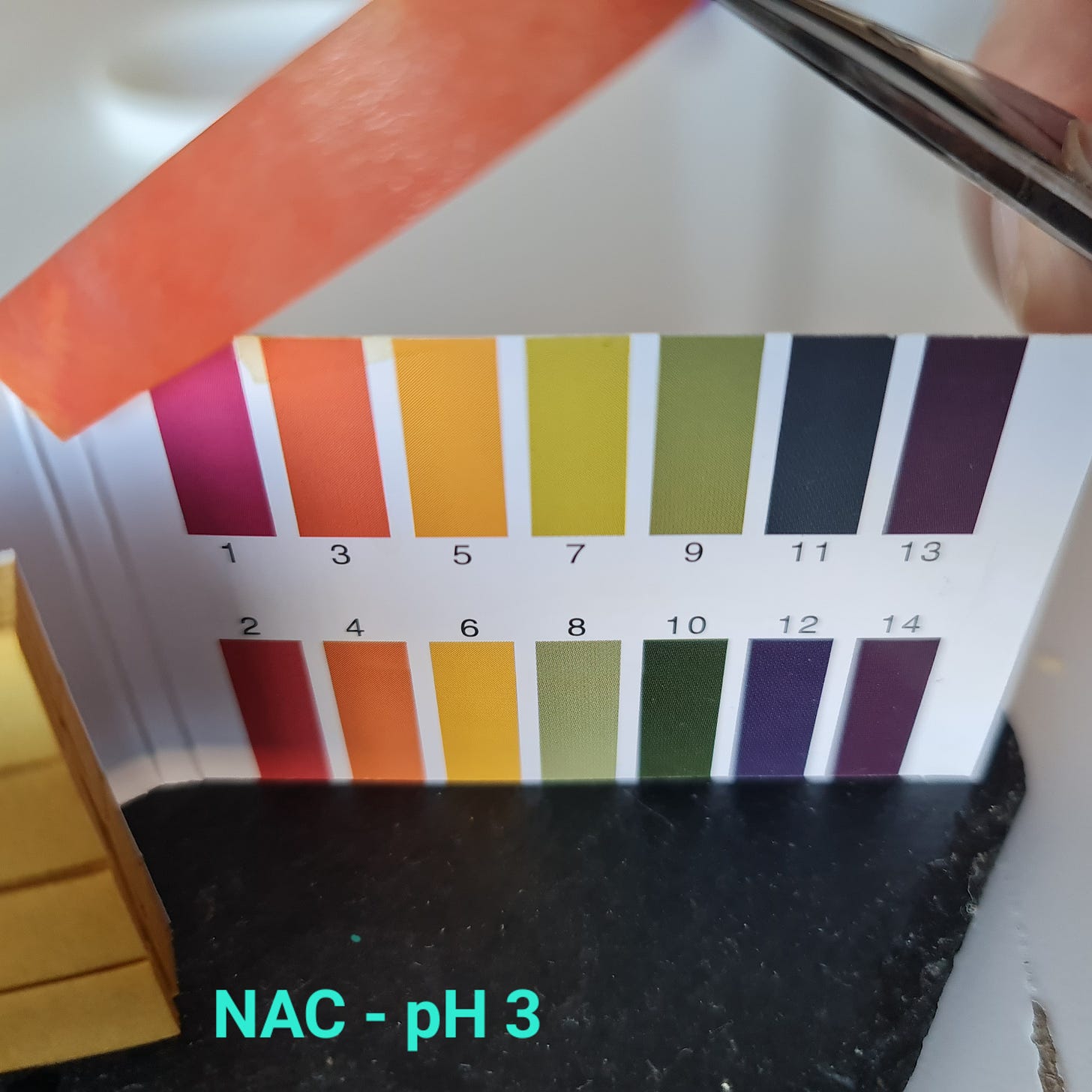
I checked the pH of the NAC solution used in the previous article:
The test results suggest that NAC is capable of affecting the net-like structure (as seen in the previous article), but it does not seem to have a significant effect on the hydrogel itself. After the successful erasure of the net, hydrogel began to develop and spread from the edges of the slide (2nd video). Additionally, the structure of some hydrogels changed (pictures), but I do not believe this caused degradation in the hydrogel, as further minor spreading was observed on the slide after 24 hours.
I am definitely very happy with the NAC’s erasing effect, as in my blood test, such a large net always develops on all sides of the slide within a few minutes:

The next posts will be about the following supplements: Nattokinase, Serrapeptase, Turpentine, Korean Pine Needle Oil.
REFERENCES:
https://www.frontiersin.org/articles/10.3389/fchem.2021.739791/full
Based on the different gelation mechanisms, hydrogels can be classified into chemical hydrogels and physical hydrogels (Mahinroosta et al., 2018).
The chemical hydrogels are cross-linked via covalent bonds, resulting in high mechanical strength, structural stability, shape memory, and irreversible properties (Liyan et al., 2018). The irreversible properties deprive them of self-healing and impair their injectability (Tu et al., 2019).
Physical hydrogels, also known as supramolecular hydrogels, could be produced by a molecular self-assembly process (Li et al., 2020; Xian and Webber, 2020; Li et al., 2021b). Molecular self-assembly relies on noncovalent interactions, such as hydrogen bonding, hydrophobic interactions, aromatic π–π stacking interactions, and electrostatic interactions, which are weak and reversible (Whitesides and Boncheva, 2002; Webber and Pashuck, 2021). The dynamic reversible nature of these interactions endows them with self-healing and shear thinning properties; hence, they are particularly suitable for biomedical applications, as they are more flexible and can be easily injected (Hu X. et al., 2020; Gupta et al., 2020; Vazquez-Gonzalez and Willner, 2020).
To form a hydrogel, the dipeptide hydrogelator first self-assembled into some kind of one-dimensional (1D) supramolecular structures, like nanofibers and twisted nanoribbons, and then into three-dimensional (3D) networks to ensnare a great number of water molecules inside (Du et al., 2015). This demands that the structure of the dipeptide hydrogelator should greatly facilitate the formation of one-dimensional assemblies.
Besides polymers, low-molecular-weight gelators (LMWGs) (generally <2000 Da) are of great interest because the associated gelators have intrinsic self-assembly ability to form different morphologies of fibers, rods, ribbons, and nanotubes…
It is worth mentioning that not all the Fmoc-modified dipeptides form hydrogels in specific conditions, and most of those dipeptides generally form hydrogels at low pH, which limited the applications in biomedical eras (Fichman and Gazit, 2014; Hendi et al., 2020; Zhang and Huang, 2021).
NAC pH
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8879903/#:~:text=In%20fact%2C%20this%20molecule%2C%20as,it%20causes%20cough%20and%20bronchospasm.
In fact, this molecule, as well as other thiol derivatives, acts mainly on the lower respiratory tract to loosen the mucus, as the main target of the drug is the mucin. However, NAC is a very acidic compound (pH 2.2), and when inhaled it causes cough and bronchospasm. (Feb 11, 2022)





Hi Keysi's... Good job well done... On my substack is the carnicominstitute.org patent Clifford Carnicom registered 31 March 2016. His Claims are there and NAC is one of them. I take it. RR
I learned recently from a friend who worked in a blood bank that when you prick your finger and get that drop of blood up you only have 5 seconds to get it on the slide with the cover glass. I had to relearn how to use my handling of slide and coverglass differently. 5 seconds makes a difference for what you see on the slide. I had to get the help of the person I was seeing to do this.